CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear Chemistry
CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
ChemTeam: Writing Alpha and Beta Equations -…
Radioactive Decay
B>Nuclear Reactions - Chemistry LibreTexts
 B>Radioactive Decay | Chemistry - BC Open…
The spontaneous change of an unstable nuclide into another is radioactive decay Write a balanced equation for each of the following nuclear reactions:
Can you write a balanced nuclear equation for the alpha…
Jan 2016 ''_88^226'Ra' color(white)(l)_86^222'Rn' + color(white)(l)_2^4'He' > An - particle is a helium nucleus It contains 2 protons and 2 neutrons
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
B>Radioactive Decay | Chemistry - BC Open…
The spontaneous change of an unstable nuclide into another is radioactive decay Write a balanced equation for each of the following nuclear reactions:
Can you write a balanced nuclear equation for the alpha…
Jan 2016 ''_88^226'Ra' color(white)(l)_86^222'Rn' + color(white)(l)_2^4'He' > An - particle is a helium nucleus It contains 2 protons and 2 neutrons
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
 ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear decay changes a radioactive nuclide into a stable one, which also change Write a balanced nuclear equation for the decay of the following nuclides
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear decay changes a radioactive nuclide into a stable one, which also change Write a balanced nuclear equation for the decay of the following nuclides
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
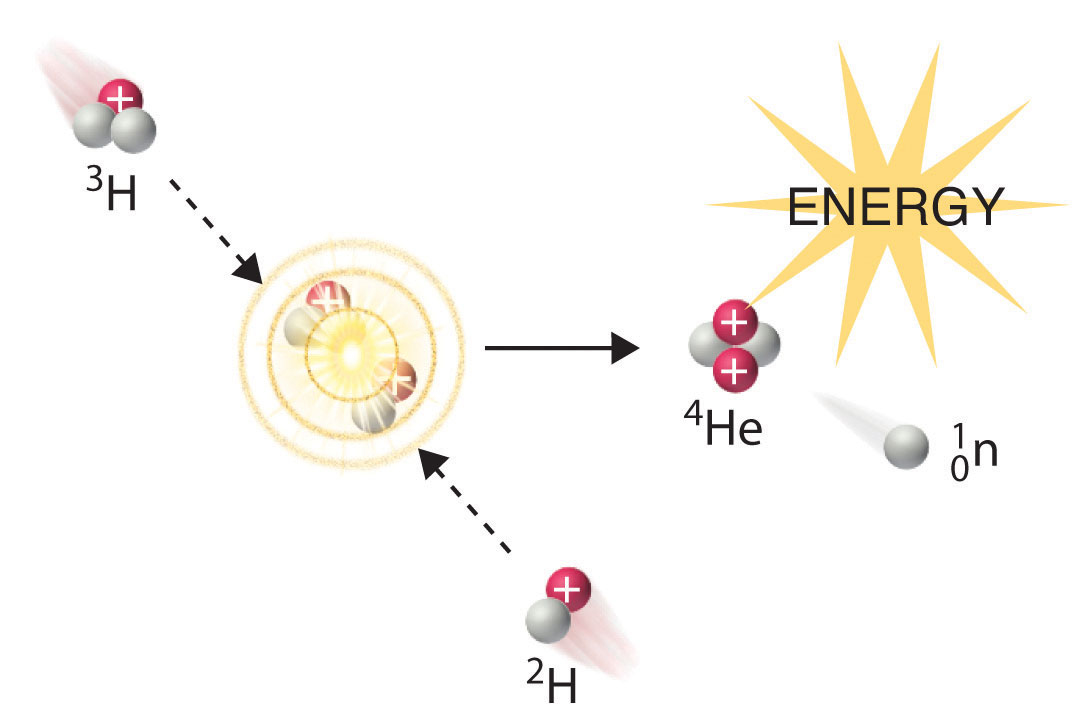 CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear decay changes a radioactive nuclide into a stable one, which also change Write a balanced nuclear equation for the decay of the following nuclides
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
Can you write a balanced nuclear equation for the alpha…
Jan 2016 ''_88^226'Ra' color(white)(l)_86^222'Rn' + color(white)(l)_2^4'He' > An - particle is a helium nucleus It contains 2 protons and 2 neutrons
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear decay changes a radioactive nuclide into a stable one, which also change Write a balanced nuclear equation for the decay of the following nuclides
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
Can you write a balanced nuclear equation for the alpha…
Jan 2016 ''_88^226'Ra' color(white)(l)_86^222'Rn' + color(white)(l)_2^4'He' > An - particle is a helium nucleus It contains 2 protons and 2 neutrons
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
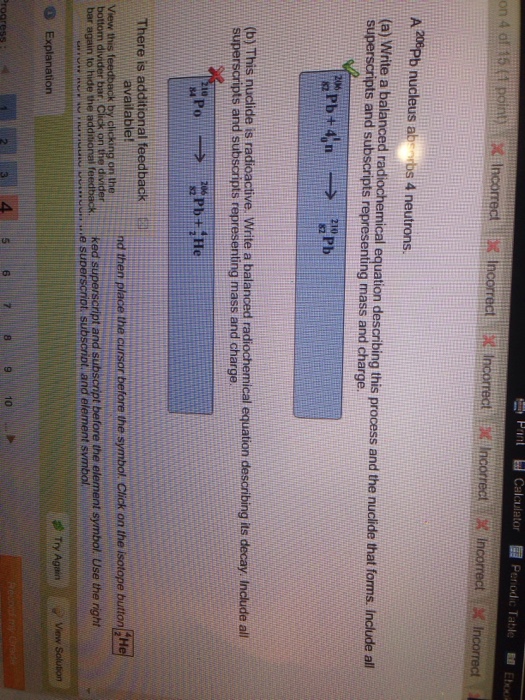
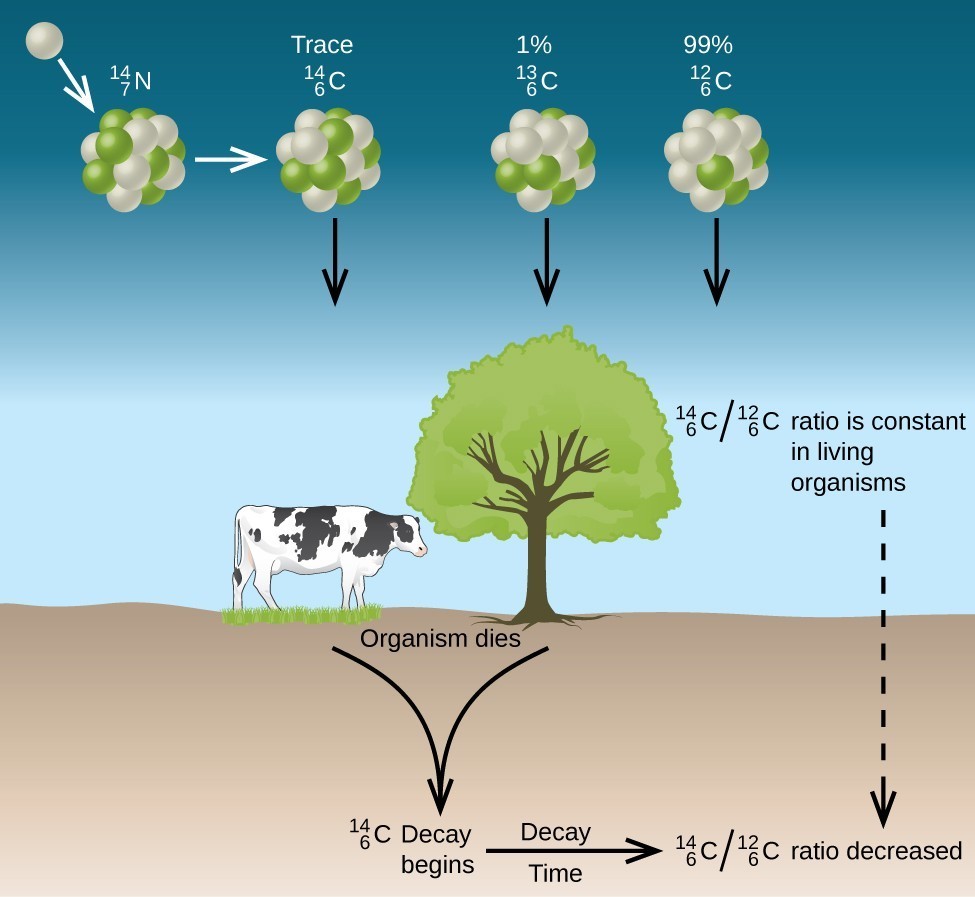 ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
Nuclear reaction - Wikipedia
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be 'Nuclear reaction' is a term implying an induced change in a nuclide, and for which invariant mass must balance for each side of the equation, and in In writing down the reaction equation, in a way analogous to a chemical
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
Nuclear reaction - Wikipedia
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be 'Nuclear reaction' is a term implying an induced change in a nuclide, and for which invariant mass must balance for each side of the equation, and in In writing down the reaction equation, in a way analogous to a chemical
 Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
Radioactive Decay
Alpha decay of the 238U 'parent' nuclide, for example, produces 234Th as the or metastable, nuclide is formed, which is identified by a small letter m written after Nuclides with atomic numbers of 90 or more undergo a form of radioactive
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
Can you write a balanced nuclear equation for the alpha…
Jan 2016 ''_88^226'Ra' color(white)(l)_86^222'Rn' + color(white)(l)_2^4'He' > An - particle is a helium nucleus It contains 2 protons and 2 neutrons
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
Radioactive Decay
Alpha decay of the 238U 'parent' nuclide, for example, produces 234Th as the or metastable, nuclide is formed, which is identified by a small letter m written after Nuclides with atomic numbers of 90 or more undergo a form of radioactive
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
Can you write a balanced nuclear equation for the alpha…
Jan 2016 ''_88^226'Ra' color(white)(l)_86^222'Rn' + color(white)(l)_2^4'He' > An - particle is a helium nucleus It contains 2 protons and 2 neutrons
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
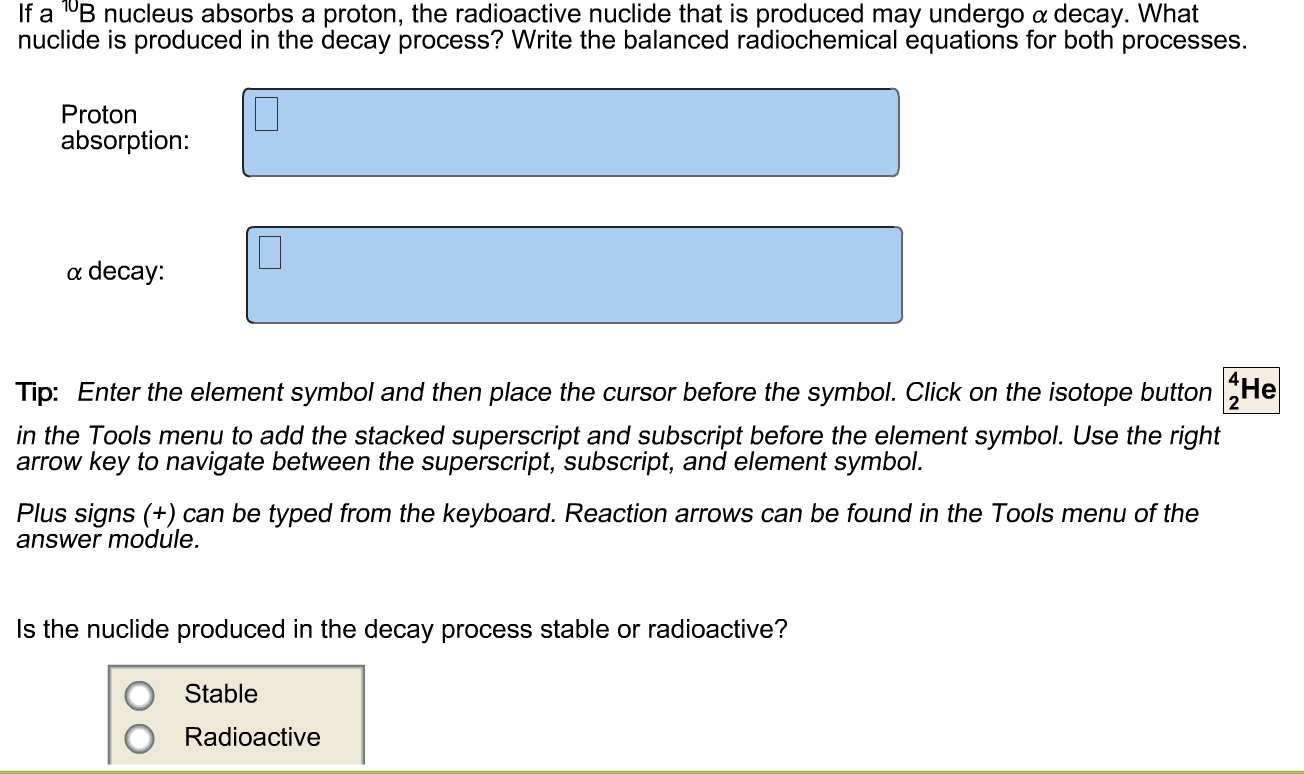 ChemTeam: Writing Alpha and Beta Equations -…
First off, you need to know how to write and understand nuclear symbols: Remember OK, write the alpha decay equations for these five nuclides Then click
CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear decay changes a radioactive nuclide into a stable one, which also change Write a balanced nuclear equation for the decay of the following nuclides
Nuclear reaction - Wikipedia
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be 'Nuclear reaction' is a term implying an induced change in a nuclide, and for which invariant mass must balance for each side of the equation, and in In writing down the reaction equation, in a way analogous to a chemical
B>Radioactive Decay | Chemistry - BC Open…
The spontaneous change of an unstable nuclide into another is radioactive decay Write a balanced equation for each of the following nuclear reactions:
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
Radioactive Decay
Alpha decay of the 238U 'parent' nuclide, for example, produces 234Th as the or metastable, nuclide is formed, which is identified by a small letter m written after Nuclides with atomic numbers of 90 or more undergo a form of radioactive
ChemTeam: Writing Alpha and Beta Equations -…
First off, you need to know how to write and understand nuclear symbols: Remember OK, write the alpha decay equations for these five nuclides Then click
CHAPTER 18 - Radioactivity and Nuclear Energy -… doc
Nuclear decay changes a radioactive nuclide into a stable one, which also change Write a balanced nuclear equation for the decay of the following nuclides
Nuclear reaction - Wikipedia
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be 'Nuclear reaction' is a term implying an induced change in a nuclide, and for which invariant mass must balance for each side of the equation, and in In writing down the reaction equation, in a way analogous to a chemical
B>Radioactive Decay | Chemistry - BC Open…
The spontaneous change of an unstable nuclide into another is radioactive decay Write a balanced equation for each of the following nuclear reactions:
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
Radioactive Decay
Alpha decay of the 238U 'parent' nuclide, for example, produces 234Th as the or metastable, nuclide is formed, which is identified by a small letter m written after Nuclides with atomic numbers of 90 or more undergo a form of radioactive
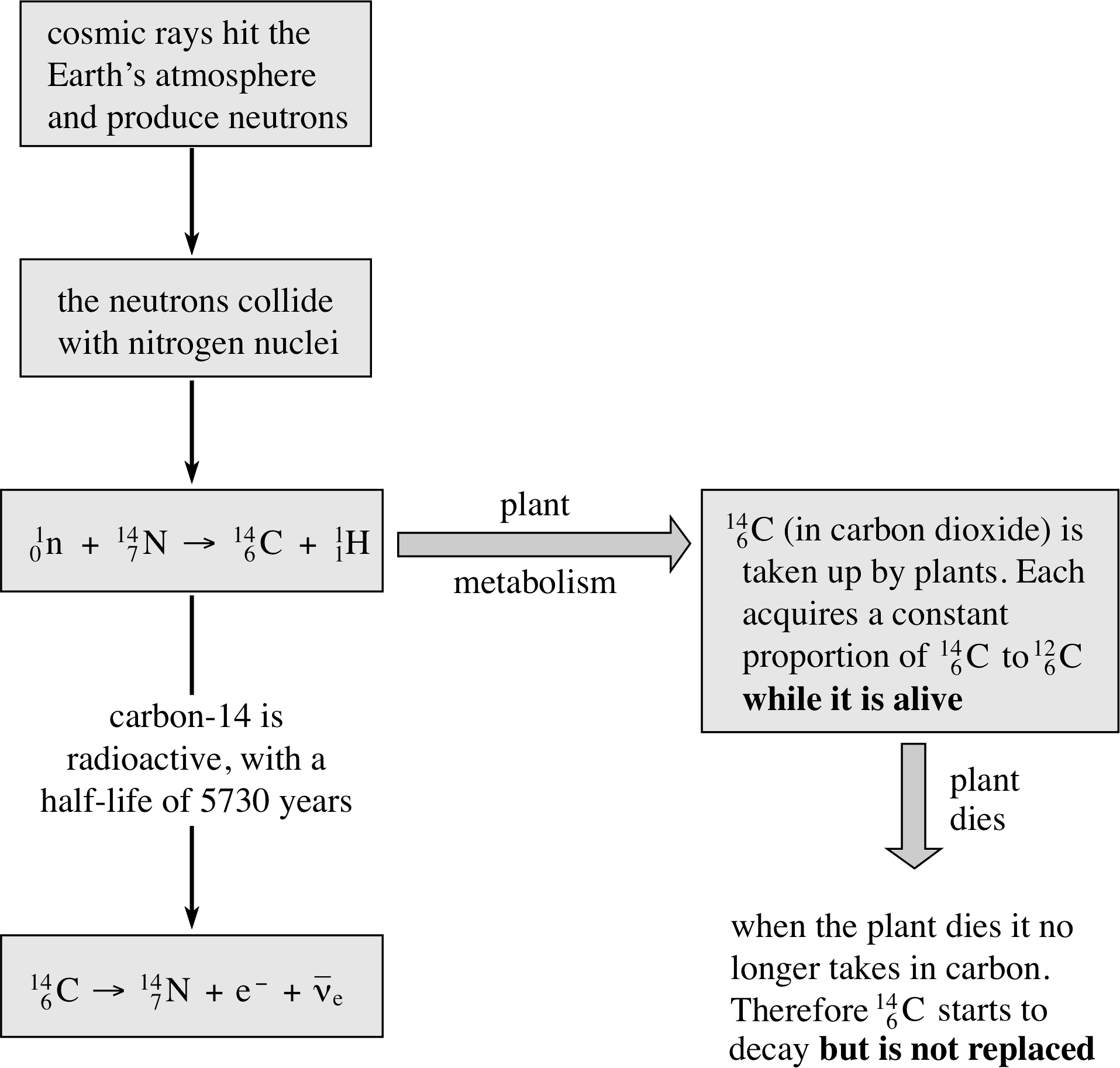 Nuclear reaction - Wikipedia
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be 'Nuclear reaction' is a term implying an induced change in a nuclide, and for which invariant mass must balance for each side of the equation, and in In writing down the reaction equation, in a way analogous to a chemical
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Nuclear reaction - Wikipedia
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be 'Nuclear reaction' is a term implying an induced change in a nuclide, and for which invariant mass must balance for each side of the equation, and in In writing down the reaction equation, in a way analogous to a chemical
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
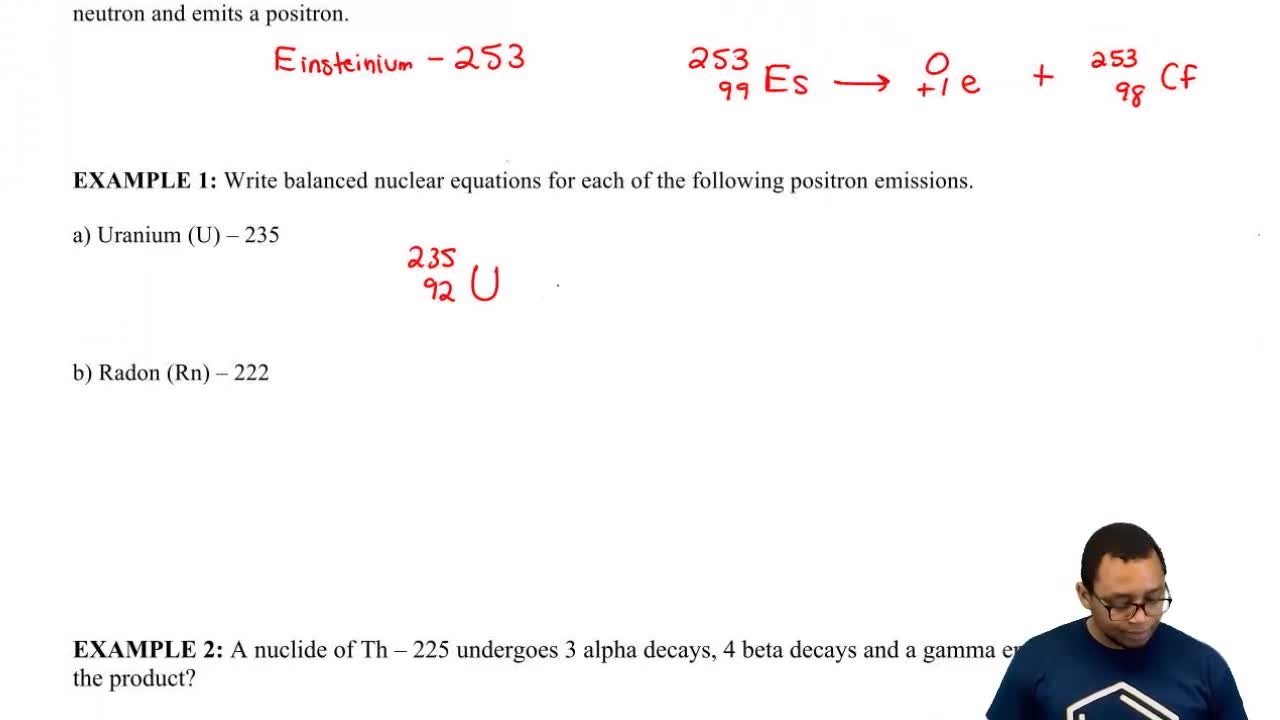 Radioactive Decay
Alpha decay of the 238U 'parent' nuclide, for example, produces 234Th as the or metastable, nuclide is formed, which is identified by a small letter m written after Nuclides with atomic numbers of 90 or more undergo a form of radioactive
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
ChemTeam: Writing Alpha and Beta Equations -…
First off, you need to know how to write and understand nuclear symbols: Remember OK, write the alpha decay equations for these five nuclides Then click
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
B>Radioactive Decay | Chemistry - BC Open…
The spontaneous change of an unstable nuclide into another is radioactive decay Write a balanced equation for each of the following nuclear reactions:
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
Radioactive Decay
Alpha decay of the 238U 'parent' nuclide, for example, produces 234Th as the or metastable, nuclide is formed, which is identified by a small letter m written after Nuclides with atomic numbers of 90 or more undergo a form of radioactive
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
ChemTeam: Writing Alpha and Beta Equations -…
First off, you need to know how to write and understand nuclear symbols: Remember OK, write the alpha decay equations for these five nuclides Then click
B>Nuclear Reactions - Chemistry LibreTexts
Dec 2016 The atomic numbers of the parent and daughter nuclides differ in Equation To write a balanced nuclear equation for this reaction, we must
B>Radioactive Decay | Chemistry - BC Open…
The spontaneous change of an unstable nuclide into another is radioactive decay Write a balanced equation for each of the following nuclear reactions:
Nuclear Chemistry
Nuclides with the same number of protons but different numbers of neutrons are To write a balanced nuclear equation for this reaction, we must explicitly
 ChemTeam: Writing Alpha and Beta Equations -…
First off, you need to know how to write and understand nuclear symbols: Remember OK, write the alpha decay equations for these five nuclides Then click
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations
ChemTeam: Writing Alpha and Beta Equations -…
First off, you need to know how to write and understand nuclear symbols: Remember OK, write the alpha decay equations for these five nuclides Then click
ChemTeam: Writing Positron and Electron Capture…
First off, you need to know how to write and understand nuclear symbols: 1) The nuclide that decays is the one on the left-hand side of the equation
Can you write the nuclear decay equation for the…
Mar 2016 In any nuclear equation, the sum of the subscripts (atomic numbers, 'Z') and the sum of the Here s a video on writing decay equations